临床试验中的单盲、双盲、三盲、破盲是什么意思?
盲法试验常用的有两种:单盲(single blinding)和双盲(double blinding),更严格的对照试验要用到三盲(triple blinding),在对照药物和试验药物剂型或外观不同时,还要用到双盲双模拟技
来源:医疗器械注册代办 发布日期:2024-06-13 阅读量:次
药物临床试验必须遵循 GCP原则、依从伦理委员会批准的试验方案; 任何有意或无意偏离或违反GCP原则和试验方案的行为叫做偏离方案(protocol deviation,PD) 或违背方案( protocol violation, PV) 。
ICH GCP 对于偏离方案的规定[2]是: 研究者/ 研究机构应当依从由申办者同意、管理当局(如有要求)批准,并已获得伦理委员会批准的试验方案。研究者/研究机构和申办者应在试验方案或类似的合同(如在美国,研究者要求签署 FDA1572 表) 上签字以确认对方案的依从。如没有和申办者达成一 致并事先得到伦理委员会的审查和书面同意,研究者不能有任何偏离方案的行为,除非必须立即消除对受试者的伤害,或只是涉及事务上的或管理方面 的变化(如监查员变更,电话号码的变更)。研究者/研究机构或申办者方面有不依从方案/SOP/现行法规的行为时,申办者应立即采取措施以保证对方案的依从。
方案偏离(Protocoldeviation,PD)
在研究者控制下的、未经IRB批准的研究方案的试验设计或程序的任何变化、分歧或者背离。
方案违背(Protocolviolation,PV)
方案违背是违反IRB批准的方案,它可影响到受试者的权益、安全性和获益,或研究数据的完整性、精确性和可靠性。
一是方案设计的原因。包括方案对入选标准或者排除标准的设计不合理;设计的随访未留时间窗或者时间窗过短;临床试验相关检查设计不合理;临床试验流程设计不合理等。
二是研究者方面的原因。主要是研究者对方案理解不深,未按照试验方案操作实施,出现检查漏开、受试者随访丢失、随机流程操作错误等情况。
三是受试者依从性差。这是临床试验操作过程中,普遍存在的问题,表现为受试者未按时回院随访、不遵医嘱服用药物或者使用医疗器械。
四是申办方的原因。因为申办方未及时提供有关资料、工具等造成的。例如器械项目中,可能出现器械缺陷未及时处理的情况。
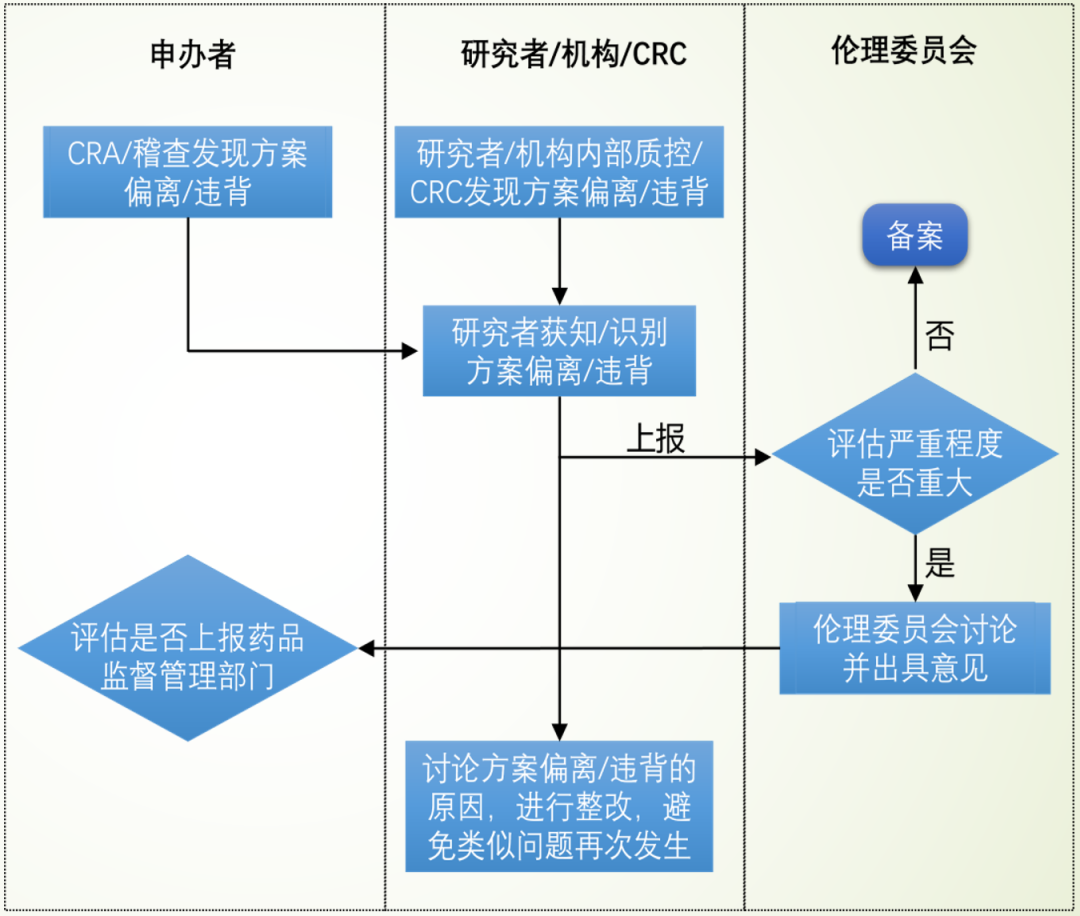
1、研究者向申办者报告;
2、研究者/申办者向伦理委员会报告;
3、向政府药品监督管理部门报告:申办者在临床总结报告中报告;申办者将重大/持续PD 发生后中止了研究者/研究机构参与试验的情况上报;研究者上报严重PD。
Tips
1、一般来说,如果是轻微 PD,CRA 要做好记录,和 PI 沟通和培训,并在监查报告上体现,上报给上级,这是监查员的职责,即①;
2、申办方汇总,定期向伦理报告,即②;
3、重大 PD,处理需要向申办方汇报,申办方如判定这是严重 PD 的话,需要通报PI 和通报伦理;视严重程度,选择进行步骤③。
Critical Protocol Deviation: A deviation from Protocol-related procedures that threatens integrity of data, adversely affects subjects and/or could invalidate acceptability of a project (or part of it). Such deviations require immediate action.
偏离方案相关程序,威胁数据的完整性,对受试者产生不利影响和/或可能使项目(或部分项目)的可接受性失效。这种偏差需要立即采取行动。
Major Protocol Deviation: A deviation from Protocol-related procedures that could affect integrity of the data or adversely affect subjects. Such deviations require timely action.
偏离方案相关程序,可能影响数据的完整性或对受试者产生不利影响。这种偏差需要及时采取行动。
Minor Protocol Deviation: A deviation from accepted procedures that will not adversely affect subjects or data integrity but should be dealt with appropriately.
偏离公认程序,不会对受试者或数据完整性产生不利影响,但应适当处理。
Must be measurable so that any person reading the information (site manager/CRA, site staff, PM/COL, Sponsor, etc.) will know exactly what is meant and root cause:
1、WHO(which Subject or investigator is involved)
2、WHAT(objectively describe the content of the protocol deviation, from a thirdpersonperspective)
3、WHEN(which visit is involved and indicate date)
4、WHY(indicate the root cause, and objectively analyze the occurrence of the protocol deviation)
5、WHERE(indicate the place where the protocol deviation occurred, such as the site number involved, the relevant department, or the subject himself, etc.)
Minor PD: Subject 1000X00X local chemical testing result for week 16 visit was reported at 12:04 am on 09Feb2022(ALT/AST results were normal), however the IP was randomly assigned at 08:33 am and administered at 09:56 am on 09Feb2022. Root cause: that was because the investigators (name) ignored a protocol requirement that dosing should be started after all safety evaluation were available.
Major PD: The subject performed the week 16 visit on 01Apr2022. Due to the error of IXRS system operation, the IP for Week12 was randomly assigned and injection to this subject. Root cause:1. Week 12 was skipped due to the impact of the epidemic, but the site staff did not register "SKIP" in the IWRS system. As a result, the visit of Week 12 was wrongly registered in the system when Week 16 visited, and drugs of Week 12 were used. 2. Sub-idid not review the IP number in a timely manner after the randomization and before the subject took the IP.
Action Items must be measurable/executable so that any person reading the information (site manager/CRA, site staff, sponsor, Auditor, etc.) will know exactly what is meant and take the action accordingly:
1、WHO (Roll or Roll+ full name) is responsible for the action?
2、WHAT exact action is required?
3、WHEN should the AI be resolved?
Example:
The new sub-Investigator, Dr. Smith (who), will need to sign the delegation log (what) as soon as possible and no later than 01Sep2013 (when).
For the Action Item owner (WHO): “site staff” or role (e.g. SC.) is acceptable.
Only name without the person role, is not acceptable Each Subject-specific Action Item must include: - Subject and visit identification - Outstanding issue and responsible party (Dr. Smith (Sub-I) or Ms. Jones (SC)
Example:
SC or Dr. Smith (Sub-I) to re-consent the Subject 100123/ABC on the latest Informed Consent (version 2.1;15Sep2013) at the subject next scheduled visit (Visit 4 on 01Oct2013).
CRA should ensure that all the PD end date is added.
PD end date is the date that deviation has ended, it can same day as PD happened date or after the PD start date.
For example:
if a PD is subject visit out of window, the PD start date should be the last planned date of this scheduled visit, and the PD end date is the actual date the subject completed visit.
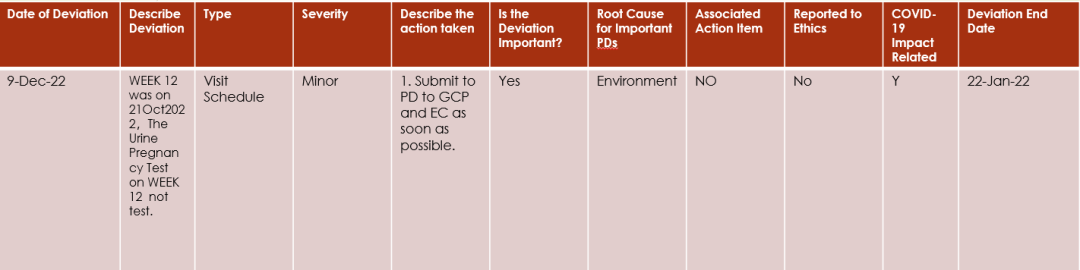
1、Start date error,The end date then precedes the start date。
2、Subject number needs to be added。
3、add how the investigator confirmed that the subject was not pregnant during the visit.
4、as per the protocol the urine/blood pregnancy test is required;
5、Type should be Laboratory Assessment
6、add EC submission expected date or specific date (DD-MMM-YYYY, or before the end of MMM-YYYY, etc.)
7、add the action on providing the relevant training to investigator;
8、Root Cause for Important PDsshould be Site Staff Error
9、Not yet submitted to EC and GCP should open an AI
10、The information needs to be updated again after completing the submission
作者:张遥

站点声明
本网站所提供的信息仅供参考之用,并不代表本网赞同其观点,也不代表本网对其真实性负责。图片版权归原作者所有,如有侵权请联系我们,我们立刻删除。如有关于作品内容、版权或其它问题请于作品发表后的30日内与本站联系,本网将迅速给您回应并做相关处理。
郑州思途医疗科技有限公司专注于医疗器械产品政策与法规规事务服务,提供产品注册备案申报代理、临床试验、体系建立辅导、分类界定、申请创新办理服务。

盲法试验常用的有两种:单盲(single blinding)和双盲(double blinding),更严格的对照试验要用到三盲(triple blinding),在对照药物和试验药物剂型或外观不同时,还要用到双盲双模拟技

刚接触CRO行业的小伙伴,在学习文件法规资料的同时,常看到一些英文类专业名词不知道是什么意思。下面,一起看看常见的临床试验专业术语: CRO行业的常用术语解释: 1:新药研发

医学的进步是以研究为基础的,这些研究在一定程度上赖于以人作为受试者的试验。--《赫尔辛基宣言》。Ⅰ期临床研究目的是确定可用于临床新药的安全有效剂量与合理给药方案。根据

SSU是Study Start Up的缩写,从最初的项目准备,到启动访视(Site Initiation Visit)之前所有的准备工作,对整个临床研究项目的启动非常关键。负责这个关键阶段工作的部门人员,就叫做SS

不知道你是否有这样的经历,去医院看病,医生开药写的都是服药中文说明。但药物临床试验相反,有些研究者喜欢写医嘱缩写,比如pc,我第一次看到就不知道什么意思,作为一个好奇
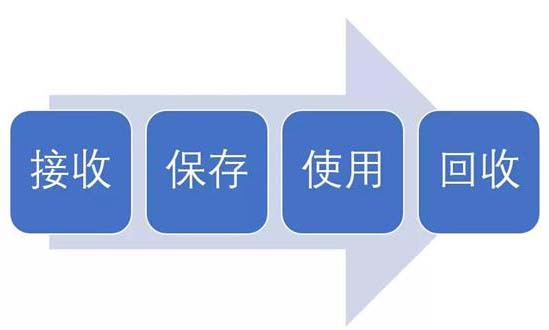
试验用药品是指用于临床试验的试验药物、对照药品。试验用药品渗透到了临床试验过程中的每一个步骤,包括药物的生产、包装、运输、保存、使用、回收等。今天我们从临床试验中

随着医疗器械出口的日益增长,根据市场的需求各医疗器械生产厂商需要符合国家和地区的质量体系法规越来越多,所以经常会碰到出处于不同法规或标准的一些比较容易混淆的概念及

在临床试验中,无论是监查员、质控人员或者项目管理人员到研究中心查看项目资料的时候,总会多多少少发现一些问题,有些问题可能大家都比较熟知,但处理手法五花八门的。处理

刚接触医疗器械CRO行业的小伙伴,在学习文件法规资料的同时,常看到一些英文类专业名词不知道是什么意思。下面,一起看看常见的医疗器械临床试验专业术语......"

不少二三类需要临床的产品,客户一听到临床报价就退缩。既然这么贵,还不如自己做......事实真的是这样吗?临床报价费用都由哪些组成?费用都谁收走了?自己做又有哪些风险?文
十年
医疗器械服务经验
联系思途,免费获得专属《落地解决方案》及报价
咨询相关问题或咨询报价,可以直接与我们联系
思途CRO——医疗器械注册临床第三方平台
